
Why Gene Therapy for Cystic Fibrosis Is Still Years Away
Developing Gene Therapy for Cystic Fibrosis Sounds Easy in Theory
Cystic fibrosis is a genetic condition that by now should have been treated with gene therapy:
First, the disease is caused by mutations in only one gene. The gene, called cystic fibrosis transmembrane conductance regulator gene, or CFTR, has been identified thirty years ago (1).
Second, all known mutations in CFTR are recessive. That means that adding a functional copy of the gene will cure the patients.
Finally, lungs are an organ that can be easily accessed. Gene therapy could be delivered to the lungs by inhalation.
Unfortunately gene delivery to the lungs turned out to be much harder than initially thought.
Molecular Mechanism of Cystic Fibrosis
Main symptom of cystic fibrosis is the formation of thick sticky mucus in the lungs. The mucus clogs airways, leading to difficulties with breathing and lung infections.
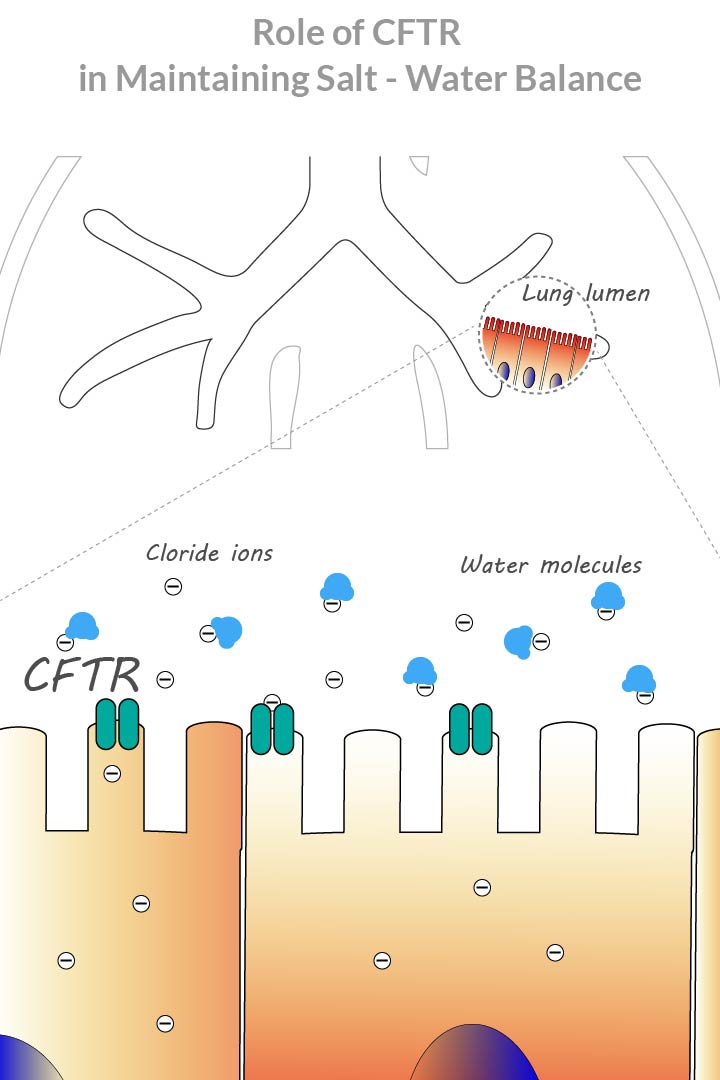
On a molecular level the disease is caused by a defect in cystic fibrosis transmembrane conductance regulator protein, also known as CFTR.
CFTR protein moves chloride ions across cell membranes to the cell surface. Chloride ions attract water, creating a salt-water balance. When CFTR protein is absent or defective, insufficient amounts of chloride ions and lack of water molecules lead to mucus formation (2).
Gene Delivery Challenge
The idea behind gene therapy for cystic fibrosis is that if we deliver a functional copy of the CFTR gene to lung cells, we would stop progression of the disease.
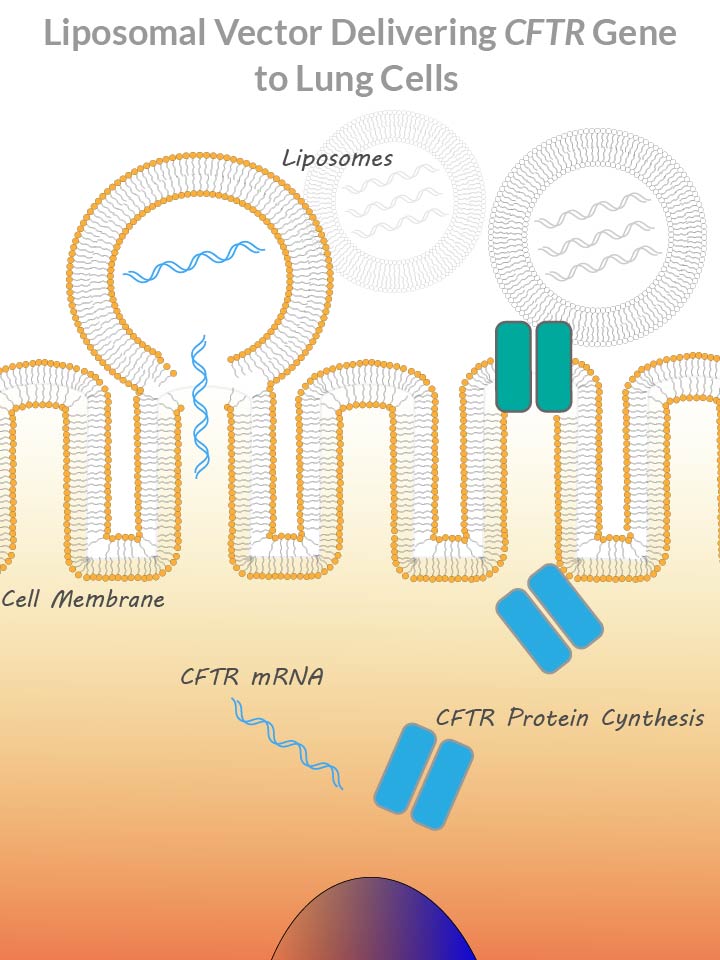
Traditionally gene delivery is done with viruses or liposomes.
Viruses have evolved to evade host organism’s defensive barriers. They are naturally designed to sneak into host cells and insert their genetic material. Viruses used in gene therapy are engineered to deliver a genetic payload, like the CFTR gene.
Liposomes are micro bubbles created from lipid molecules. During liposome formation, payload genetic material is incorporated inside the bubble. After a liposome fuses with a cell membrane, it releases its contents inside cell cytoplasm.
The biggest challenge with cystic fibrosis gene therapy is a layer of mucus on the inner surface of the lungs. The mucus prevents gene delivery vehicles from making contact with lung epithelial cells.
One of the main goals of gene therapy research is design of gene delivery vehicles that can work well in cystic fibrosis patients (3, 4).
The Clinical Trials
Currently the most promising gene therapy for cystic fibrosis is MRT5005 developed by Translate Bio (5).
MRT5005 aims to deliver CFTR messenger RNA to lung epithelial cells. Translate Bio does not elaborate on how they do mRNA delivery, other than saying that it is done through nebulization. Nebulization is a way of drug delivery to the lungs that involves creating a mist inhaled by a patient.
MRT5005 is in Phase 1 clinical trials. It requires 5 – 10 years of testing before it could reach patients.
Considering average drug failure rates of 50% for each of phase II and phase III stages, there is a hypothetical 25% chance that MRT5005 will make it.
There are three gene therapy treatments that are in preclinical trial stage (6).
LUNAR-CF developed by Arcturus Therapeutics also aims to deliver CFTR mRNA. LUNAR stands for “Lipid-enabled and Unlocked Nucleomonomer Agent modified RNA”, meaning that it uses a proprietary liposome based RNA delivery mechanism.
Two more preclinical trials of gene therapies for cystic fibrosis are done by Spirovant and 4D Molecular Therapeutics. Both companies use AAV based vectors to deliver CFTR to the lungs.
The chances of success of preclinical trials are even harder to predict. All we could tell today, is that research keeps moving on.
Advantages of Gene Therapy
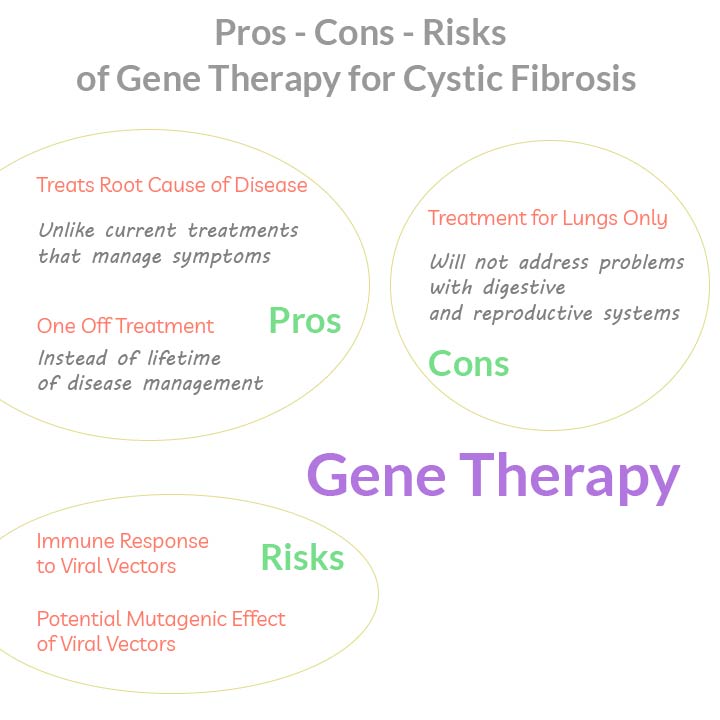
The main advantage of gene therapy approach is that it deals with the root cause of the disease.
Traditional treatment of cystic fibrosis involves using antibiotics that treat lung infections, and removal of mucus. Both approaches do not address the underlying cause of the disease, and therefore need to be repeatedly applied during the life of a patient.
Cystic fibrosis is also a progressive condition, meaning that it gets worse over time. Most patients have shorter-than-average life expectancy and may die because of lung failure.
Gene therapy promise is that one day kids born with cystic fibrosis would get a one-off treatment and live a normal life like everyone else.
Hidden Problems
The main problem with gene therapy for cystic fibrosis is that it is not here yet. It is years away from being offered to patients.
There is also an unspoken flaw hidden in the design of therapies that are being developed. They are focused on the lungs only. Once the lungs are cured, the patients would still be suffering from mucus build up in the pancreas, in the liver, and the male reproductive system.
Third problem with gene therapy is that it can stop the progression of the disease, but it will not reverse it. It will be an almost ideal solution for kids. It will be less ideal for adults in whom disease has already progressed.
Gene Therapy Risks
Biggest risks of gene therapy come from using viral vectors for gene delivery.
Human body’s immune system considers viruses as intruders, and initiates a response against them. In severe cases immune response could lead to inflammation and organ failure.
Jesse Gelsinger was a patient who died during early gene therapy trials for a different condition. He had a massive immune response likely triggered by a viral vector (7).
Some viral vectors insert their DNA into host chromosomes, which could lead to disruption of native genes. This effect is called insertional mutagenesis. In a different case of extreme gene therapy failure, viral integration lead to disruption in a tumor suppressor gene. As a result, several of the patients receiving the therapy developed leukemia (8).
A lot of work in gene therapy is focused on developing safer viral vectors. For example, vectors based on adeno-associated viruses produce low immune response. AAV based vectors also do not integrate into the host genome, minimizing the risk of insertional mutagenesis.
MRT5005, currently the most promising gene therapy for cystic fibrosis, is likely to be based on a non-viral vector. Although Translate Bio does not elaborate on their gene delivery technology, the fact that it delivers messenger RNA implies that they use liposomes as vectors.
If MRT5005 makes it to the market, it will be a low risk solution.
Projected Cost of Gene Therapy for Cystic Fibrosis
Without any treatment on the market, we can only speculate about the expected cost of gene therapy for cystic fibrosis.
Here is a back-of-the-envelope calculation of a possible price tag for cystic fibrosis gene therapy.
There are three factors driving up the price of gene therapy. First one is the cost of a therapy development. It costs between $1B (that’s a million with three more zeros) and $5B for a company to pass all regulatory approvals before a drug is brought to patients.
Second factor is small market size. Treatments for rare diseases can be sold to a small number of patients. With high development and regulatory costs, a pharmaceutical company needs to charge a lot from each patient to make their money back.
Third factor is the cost of production. It is much harder to produce viral particles packaged with DNA than to produce chemical molecules.
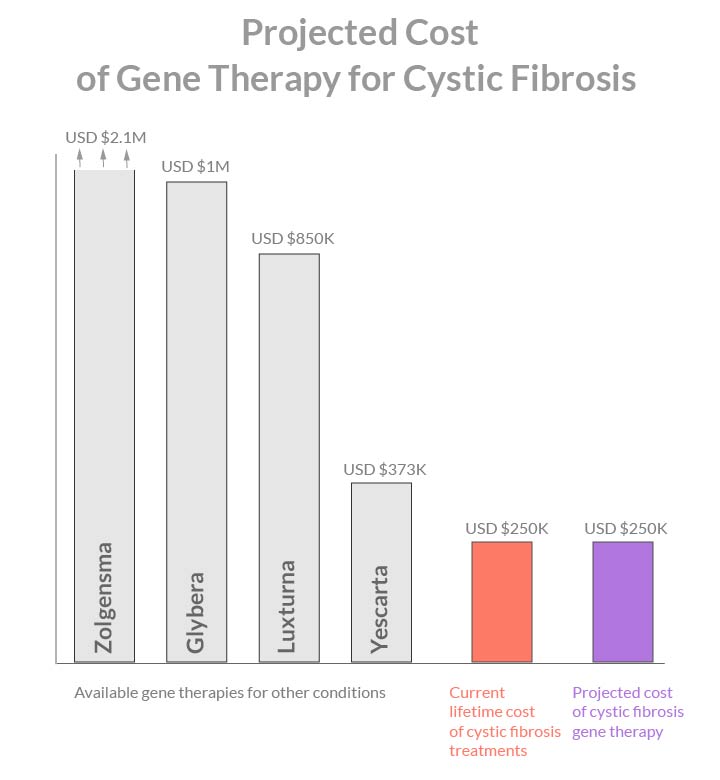
Current gene therapy treatments range in price from USD 2.1M for Zolgensma, $1M for Glybera, $850K for Luxturna, and down to $373K for Yescarta.
The good news is that technology becomes cheaper with time.
Regulatory process will get smoother with each new therapy entering the market.
Production of viral particles can be streamlined. Non-viral gene therapies based on nanoparticles are developed for the exact same reason of reducing production cost. Finally, cystic fibrosis is not as rare as some other rare diseases, so there will be a fair demand for the therapy once it becomes available.
My estimate is that in five to ten years time, when cystic fibrosis gene therapy becomes available, it will cost below $300K. It will be comparable to the current lifetime cost of hospital admissions and medications for this condition.
Ethical Issues
Unique ethical issue with cystic fibrosis gene therapy involves recruiting children to clinical trials (9).
On one hand, there is a sufficient number of adult patients that can be recruited into therapy studies. It is not critical for therapy development to conduct studies in children, and to expose them to potential harm.
On the other hand, if a treatment provides hope for potential benefit, it may be unethical to deny children access to such treatment.
From a scientific viewpoint, gene therapy could be more effective in children than in adults. Children with cystic fibrosis are born with normal lungs. Although children develop lung conditions at an early age, their lungs could be more susceptible to therapy intake. In comparison, in adults the buildup of mucus is deemed the main obstruction to efficient gene delivery.
A lot of ethical considerations revolve about explaining potential risks and benefits, and obtaining informed consent from parents or children themselves.
Current clinical trials for MRT5005 limits participation to adults 18 years or older (10).
References
- Science. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA
- Gene Therapy. Beyond cystic fibrosis transmembrane conductance regulator therapy: a perspective on gene therapy and small molecule treatment for cystic fibrosis
- Advanced Drug Delivery Reviews. Gene transfer to the lung: lessons learned from more than 2 decades of CF gene therapy
- Human Molecular Genetics. Advances in gene therapy for cystic fibrosis lung disease
- Translate Bio. Pipeline. Present and future focus
- Cystic Fibrosis Foundation. Drug development pipeline
- CMAJ. Death but one unintended consequence of gene-therapy trial
- Molecular Therapy. Gene therapy for SCID-X1: round 2
- Journal of Medical Ethics. Gene therapy for children with cystic fibrosis – who has the right to choose?
- Cystic Fibrosis Foundation. RESTORE-CF: Phase 1/2 study of MRT5005 drug in adults with cystic fibrosis (Parts A, B and B Expansion) (Translate Bio MRT5005-101)