
AAV Gene Therapy – Current Gene Delivery System of Choice
What Makes Adeno-Associated Virus so Special
Adeno-associated virus – or AAV – has been used in 100+ gene therapy clinical trials (1).
Two out of four gene therapy products currently approved by the FDA use AAV for gene delivery (2). They are Luxturna and Zolgensma.
The AAV is unique because:
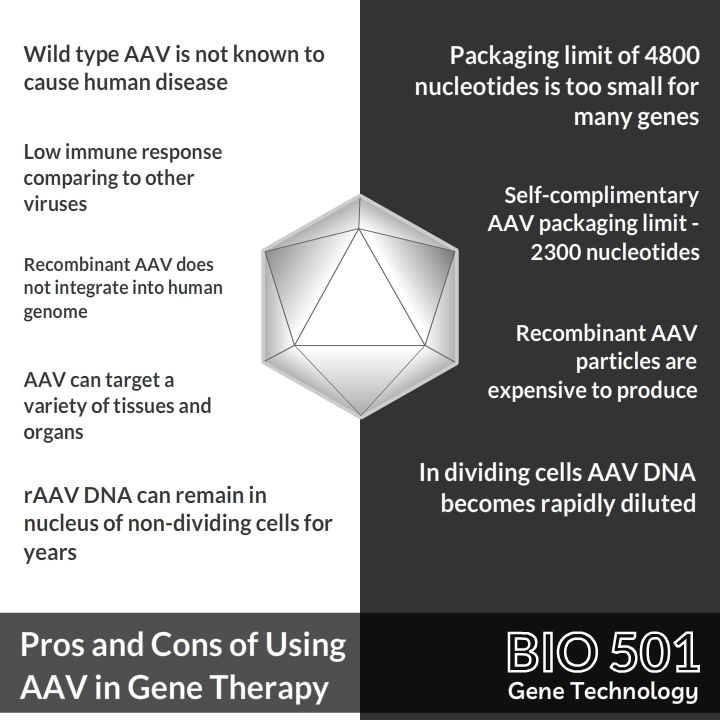
- This virus is not known to cause any human disease
- It can deliver its genetic material to a variety of tissues and organs
- Recombinant AAV used in gene therapy does not integrate into the human genome. Meaning there is no risk of activating a cancer gene
- Genetic material delivered by AAV can remain in the nucleus of non-dividing cells for years
On the flip side:
- The AAV can only package DNA molecules up to 4800 nucleotides long, limiting its usage to small genes
- When cells divide, only one of them inherits AAV genetic material. In dividing cells AAV DNA becomes rapidly diluted
- Recombinant AAV particles are expensive to produce (3)
How Does AAV Gene Therapy Work
Gene therapy is all about gene delivery.
Zolgensma delivers the SMN1 gene to motor neurons. Luxturna delivers the RPE65 gene to retinal cells. Glybera delivers the LPL gene to muscles.
Viral particles are ideal vehicles for delivering genes. Viruses have naturally evolved to bypass obstacles between the external environment and cell nucleus. They sneak past the immune system, cross cell membranes, and travel through nuclear pores.
AAV particles are made of 60 protein subunits that form a viral shell. Hidden inside the shell is a piece of single stranded DNA. The DNA carries a set of viral genes flanked by short regions called Inverted Terminal Repeats, or ITRs (3).
The cool thing about AAV is that the DNA sitting between ITRs is replaceable. We can take a gene of interest, surround it with ITRs, package it inside a viral shell, and have a ready AAV-based gene delivery vehicle.
AAV made of ITRs and a gene of interest is called recombinant AAV, or rAAV.
Once inside the nucleus, DNA from rAAV forms an episome. It remains on its own without integrating into cell chromosomes. rAAV DNA starts producing messenger RNA, which in turn produces proteins that restore metabolic balance in the organism.
Use of AAV2 and AAV9 Serotypes in Gene Therapy
Serotype is a term from viral taxonomy.
Within one viral species, there are subgroups that differ by subtle structure of their surface proteins. AAV serotype is a subgroup of AAVs that can bind to certain receptors on the cell surface.
The most studied serotype of AAV is AAV2. AAV2 can bind to a broad range of cell surface receptors, and infect a variety of tissues. There have been 40 gene therapy clinical trials that used AAV2 based vectors (4). Luxturna is an AAV2-based gene therapy that made it to the market (5).
AAV9 is a serotype that has been isolated from human liver tissue. The most attractive feature of AAV9 is its ability to cross the blood brain barrier. AAV9 can infect the cells of the central nervous system, including primary neurons (3).
Zolgensma, the gene therapy for spinal muscular atrophy, is based on AAV9. In babies under two years old Zolgensma is administered by intravenous injection. AAV9 particles of Zolgensma travel to the brain, cross the blood brain barrier, infect motor neurons, and restore their function (6).
The Safety of AAV
AAV appears to be the safest out of all the viruses used for gene therapy.
There are three factors that contribute to AAV safety. First, the wild type AAV does not appear to cause any human disease. It even can not replicate on its own without helper viruses.
Second, AAV is the least immunogenic. Immune response to a viral vector could lead to serious complications for a patient. Death of Jesse Gelsinger during a clinical trial occurred because of the immune response to adenoviral vector (7).
Most people have been exposed to wild type AAV during the course of their lives. Their immune system can recognise one or several AAV serotypes. Yet, in the case of AAV, the immune response is usually discussed as a factor affecting the efficiency of gene therapy, not as a risk factor for the patient (3).
Third factor of AAV safety is that recombinant AAV does not integrate into the human genome.
Viral integration is a risk factor in gene therapy, because it can disrupt or activate unknown genes. Another tragic event in the history of gene therapy was activation of cancer genes in children treated for severe combined immunodeficiency with a retroviral vector (8).
During construction of recombinant AAV vectors, genes responsible for viral integration are removed and replaced with therapeutic DNA payload. The only “viral” component of DNA that makes it to the cell nucleus are inverted terminal repeats. As a result, rAAV DNA settles in the nucleus on its own as an episome. It does not integrate into the chromosomes.
Manufacturing of AAV Particles
rAAV is produced in cell culture, either in human HEK 293 or insect Sf9 cell lines.
Production of rAAV requires transfecting cells with three different genetic constructs: construct 1 carrying a transgene payload surrounded by ITRs, construct 2 with AAV Rep and Cap proteins, and construct 3 that provides helper genes isolated from adenovirus (3).
Generating and maintaining cell lines that express all three constructs simultaneously is a challenging process.
Another challenge is that after production rAAV particles need to be separated from cell leftovers, from empty AAV capsids, and from particles of the helper virus.
Small batches of rAAV used in preclinical and early stage clinical trials have been traditionally produced by researchers in adherent cell cultures. Then the rAAV particles were purified by ultracentrifugation. Neither adherent cell cultures, no ultracentrifugation are scalable. The moment a gene therapy enters a larger scale clinical trial, or enters the market, problems with rAAV manufacturing inevitably kick in.
Currently there is a trend for development of bioreactors and chromatography-based purification methods that will allow for large scale production of GMP grade rAAV (9,10).

The Most Promising AAV Gene Therapy Clinical Trials
There are five Phase 3 clinical trials that use rAAV for gene therapy. It is likely that some of those trials would lead to approved products entering the market in the coming years.
AMT-061 is an AAV5-based gene therapy for Hemophilia B being developed by uniQure. uniQure is the company that developed Glybera, the first ever gene therapy approved in Europe or the United States. AMT-061 is showing good promise in terms of both safety and effectiveness (11,12).
BIIB111 is a treatment for choroideremia, a vision disorder. It is being developed by Biogen. BIIB111 aims to deliver Rab-escort protein 1, or REP1, to retinal cells. There are currently 169 participants involved in the clinical trial that is spread across 14 sites in the US and Europe. Approval of Luxturna, a gene therapy for a different inherited retinal disease, is likely to simplify both approval process and public acceptance of BIIB111 (13,14).
BMN270 is a gene therapy treatment for Hemophilia A developed by BioMarin. BMN270 aims to deliver blood clotting factor VIII using AAV5 based vector. Phase 2 clinical studies for BMN270 showed promising results with reduced bleeding and reduced usage of injectable factor VIII by the patients. Phase 3 trials of BMN270 are expected to be completed by March 2024 (15,16).
LYS-SAF302 is a gene therapy for mucopolysaccharidosis type IIIA (MPS IIIA) being developed by Lysogene, a Paris-based biotechnology company. A February 25, 2020 announcement by the company says that they received the USA FDA Fast Track designation for their MPS IIIA gene therapy (17,18).
GS010 is an AAV2-based gene therapy for Leber’s hereditary optic neuropathy, or LHON. GS010 is being developed by GenSight Biologics, also a company based in Paris. There are 90 patients involved in a study that is due for completion in June 2021. The study outcomes are promising. GenSight is planning to file for GS010 approval in Europe in the third quarter of 2020 (19,20).
References
- ClinicalTrials.gov
- U.S. Food and Drug Administration
- BioDrugs. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy
- ClinicalTrials.gov
- Luxturna – Mechanism of Action
- Zolgesma
- SMAJ. Death but one unintended consequence of gene-therapy trial
- J Clin Invest. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1
- Muscle Gene Therapy. Large-Scale Clinical Manufacturing of AAV Vectors for Systemic Muscle Gene Therapy
- Vigene Biosciences. AAV Packaging
- ClinicalTrials.gov NCT03569891
- uniQure
- ClinicalTrials.gov NCT03496012
- BioGen
- ClinicalTrials.gov NCT03370913
- BioMarin https://www.biomarin.com/products/pipeline/bmn-270/
- ClinicalTrials.gov NCT03612869
- BioSpace
- ClinicalTrials.gov NCT03293524
- GenSight Biologics